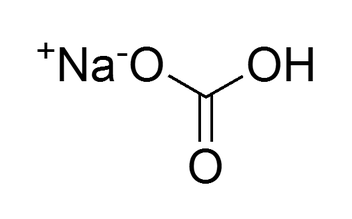
Molecular Structure
Percentage Composition by Mass:
Na = 27.36 %
H = 1.20 %
C = 14.30 %
O = 57.14 %
Type of Bonding:
Sodium Bicarbonate is a polar compound. The sodium (Na) creates a + charge, and the bicarbonate (HCO3) will create a - charge.
This is an ionic bond.
Chemical Properties:
NaHCO3 will melt at 50 degrees Celsius, and boils at 851 degrees Celsius.
In its natural state, it has a density of 2.20 g mol.
Reaction tendencies:
Sodium Bicarbonate reacts with acids and bases as a neutralizer. Humans have sodium bicarbonate in our stomachs to neutralize the stomach acids.
Na = 27.36 %
H = 1.20 %
C = 14.30 %
O = 57.14 %
Type of Bonding:
Sodium Bicarbonate is a polar compound. The sodium (Na) creates a + charge, and the bicarbonate (HCO3) will create a - charge.
This is an ionic bond.
Chemical Properties:
NaHCO3 will melt at 50 degrees Celsius, and boils at 851 degrees Celsius.
In its natural state, it has a density of 2.20 g mol.
Reaction tendencies:
Sodium Bicarbonate reacts with acids and bases as a neutralizer. Humans have sodium bicarbonate in our stomachs to neutralize the stomach acids.